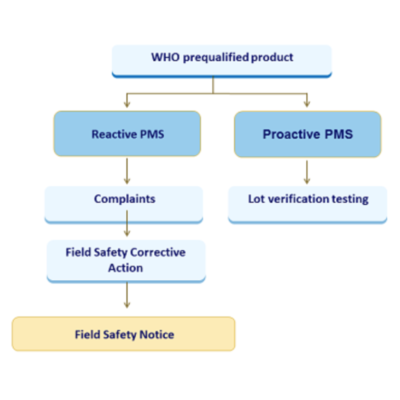
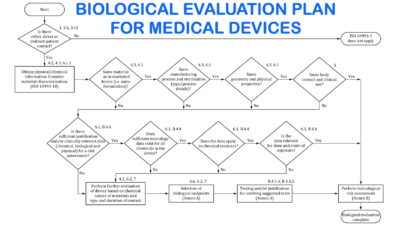
BIOMEDRIC, which started its activities in 2020, is dedicated to the metrics of biomedicine on medical devices (MD) and in vitro diagnostic medical devices (IVDMD). In this context, specifically, we aim to meet the scientific/technical/regulatory consultancy, reporting, and filing requirements of MD and IVDMD manufacturers, which has become a major need, especially with the new European Union Regulations on MD (Regulation (EU) 2017/745)) and IVDMD (Regulation (EU) 2017/746), as well as with the tendency of the FDA regulations. For this purpose, by eliminating the waste of time and money with accurate and fast solutions thanks to our strong scientific and technical expertise, we are providing high-quality scientific/technical/regulatory support from preclinical to post-clinical stages for MD and IVDMD manufacturers to translate their products into the clinic.
Please contact us for the high-quality scientific/technical/regulatory consultancy, reporting, and filing services you may need.